This blog is part of our Medicaid Access and Managed Care Rules for HCBS series, which examines the implications of the new rules on home and community-based programs and provides states with suggestions to improve data, quality reporting, and oversight.
The Centers for Medicare & Medicaid Services’ (CMS’) final rule for Ensuring Access to Medicaid Services (Access Rule) requires states to use and integrate a broad array of data sources. In our experience working with states, these data often live across multiple state agencies. Now, the Access Rule—along with the home and community-based services (HCBS) components of the Medicaid and Children’s Health Insurance Program (CHIP) Managed Care Access, Finance, and Quality Rule (Managed Care Rule)—signals that we should think about these data more holistically and state agencies will need to carefully map out their data itinerary to meet this goal.
We’ve already covered ways that states should begin thinking about responding to the new Access Rule and managed care rules and how to prepare using existing HCBS Medicaid program enrollment and service utilization data. Here are the data sources that state Medicaid agencies will collect, analyze, and report on the road to Access Rule compliance, and how to begin preparing for the journey:
- Medicare data for dually eligible HCBS users. States that operate Money Follows the Person (MFP) programs will be required to report on several HCBS Quality Measures by 2026. All states will be required to do so by 2028 as part of the Access Rule. Three measures require states to use Medicare data to calculate the measures for people who are dually eligible. These measures are MLTSS/LTSS-6, -7, and -8: they examine whether people using HCBS go on to be admitted to long-term care institutions (MLTSS/LTSS-6) and if people living in these institutions for a short stay (MLTSS/LTSS-7) or long stay (MLTSS/LTSS-8) successfully move back to their communities. As part of these measures, “institution” includes Medicare-paid nursing facility stays. Successful discharge or transition assesses acute hospitalizations and nursing facility readmissions in which Medicare is the first payer for people who are dually eligible. States that are not already routinely using Medicare data for monitoring can seek assistance with obtaining these data though the State Data Resource Center (SDRC). In some cases, states may already be receiving Medicare data from the SDRC, so creating the roadmap may mean finding and coordinating with state staff already using those data.
- Data from a newly required fee-for-service grievance system. States that deliver HCBS through managed care are required to ensure their managed care plans have systems to field, investigate, adjudicate, and report on grievances—meaning the expressions of dissatisfaction for reasons other than denial of services or payment—that fall outside the state fair hearing process. The new rule adds a parallel requirement for HCBS delivered as fee-for-service (FFS) and specifies minimum requirements for the system that must be in place by 2026. States are encouraged to structure the data collected on the FFS grievance process to enable tracking and trending on populations, services, and topics of interest and align those data with reporting in managed care, where both sets of data exist. Mathematica developed an issue brief for CMS that offers an example typology (see Figure 8) to structure this reporting.
- Data from case management reports. States will need case management data for two reporting requirements described in the Access Rule. These data are needed for: (1) measures in the HCBS Quality Measure Set (for example, MLTSS/LTSS-1 and -2) that assess timeliness of comprehensive assessments and care planning, and (2) to report on the timeliness of functional need reassessments and person-centered plans. Reporting begins as early as 2026 on the HCBS Quality Measures for MFP recipients (with all states reporting by 2028) and by 2027 for person-centered reporting metrics. To prepare, states should begin reviewing how their existing case management data map to the required measure elements and assess the feasibility for reporting. States that do not have central standardized case management systems for all their HCBS programs must consider how collected data may differ across managed care organizations or agencies operating HCBS programs and how these forks in the road may affect reporting.
- Critical incidents. The Access Rule requires states to update their critical incident management systems by establishing a standardized, minimum definition for critical incidents. States must also require the entities responsible for investigating these incidents to report on their status and resolution by 2027. And by 2029, these data must be incorporated into an electronic system that can identify, report, triage, investigate, resolve, track, and trend these incidents. To do this well, states must gather data from a variety of sources, including their own Medicaid Management Information Systems (for claims and program integrity information) and from other relevant agencies (such as adult and child protective services). Many states will be able to build on the existing critical incident systems infrastructure to meet these requirements. When existing systems are not well aligned with new requirements, however, the road to compliance may be difficult. CMS estimates this requirement will be the second most costly to implement, of items in the rule.
- Waiver waiting list enrollment and duration. By 2027, states must report annually on how they maintain waiting lists for their Section 1915(c) waivers, whether and how frequently the state screens and rescreens people on the waiting lists for waiver program eligibility, the number of people on waiver waiting lists, and the average amount of time that people who newly enroll on waiting lists in the past year spend on them before receiving services. Depending on how states currently organize and manage waiting lists for 1915(c) waivers, they may need to change policies, workflows, or both to meet the reporting requirements. For example, if a state does not periodically rescreen an individual following their waitlist entry, the state will need to decide whether to make this policy change to the requirement to report these data. These changes can lengthen the journey, due to the time to seek review and approval from CMS for waiver amendments. To make these newly available waitlist data meaningful for community members and other interested parties states are also encouraged to make them publicly available.
- Home care and habilitation provider payment adequacy information. States must begin reporting on the percentage of compensation that goes to home care providers (that is, personal care, home health aide, and homemaker providers) and to habilitation providers by July 2028. The goal of this rule is to ensure that 80 percent of Medicaid reimbursement is used for direct care worker compensation by 2030. Though the rule provides specific calculation instructions, the providers who must generate these reports and, if applicable, the managed care plans that will pass them through to states will likely require clear, standardized reporting templates and thorough validation. States should prioritize planning for this requirement because CMS estimates that it will be the costliest item to implement in the rule.
Milestones for HCBS Access Rule Compliance
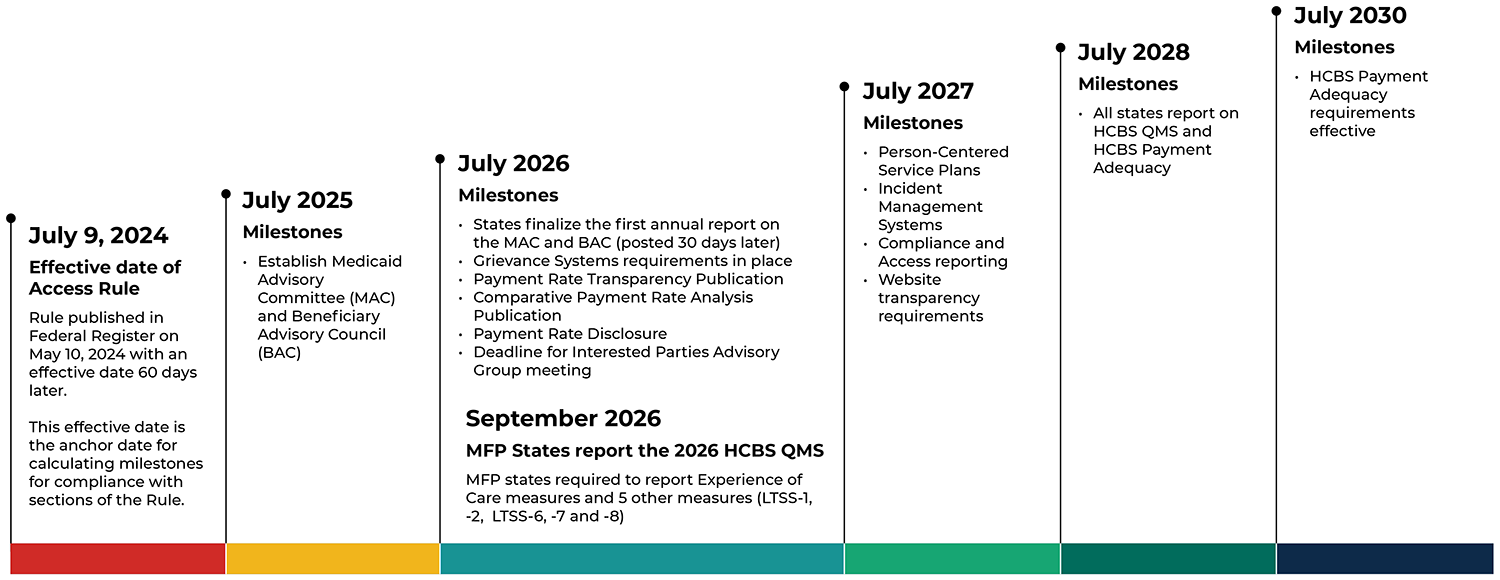
Identifying and extracting data from different state systems can take longer than expected, so this summer is an ideal time to identify and plan for visits with these data owners. The road to Access Rule compliance will stretch over the next several years, and efforts to coordinate now on the necessary stops will smooth the journey towards improved monitoring of HCBS systems.